Hepatitis B Birth Dose Sparks Debate as CDC Weighs Vaccine Policy Changes
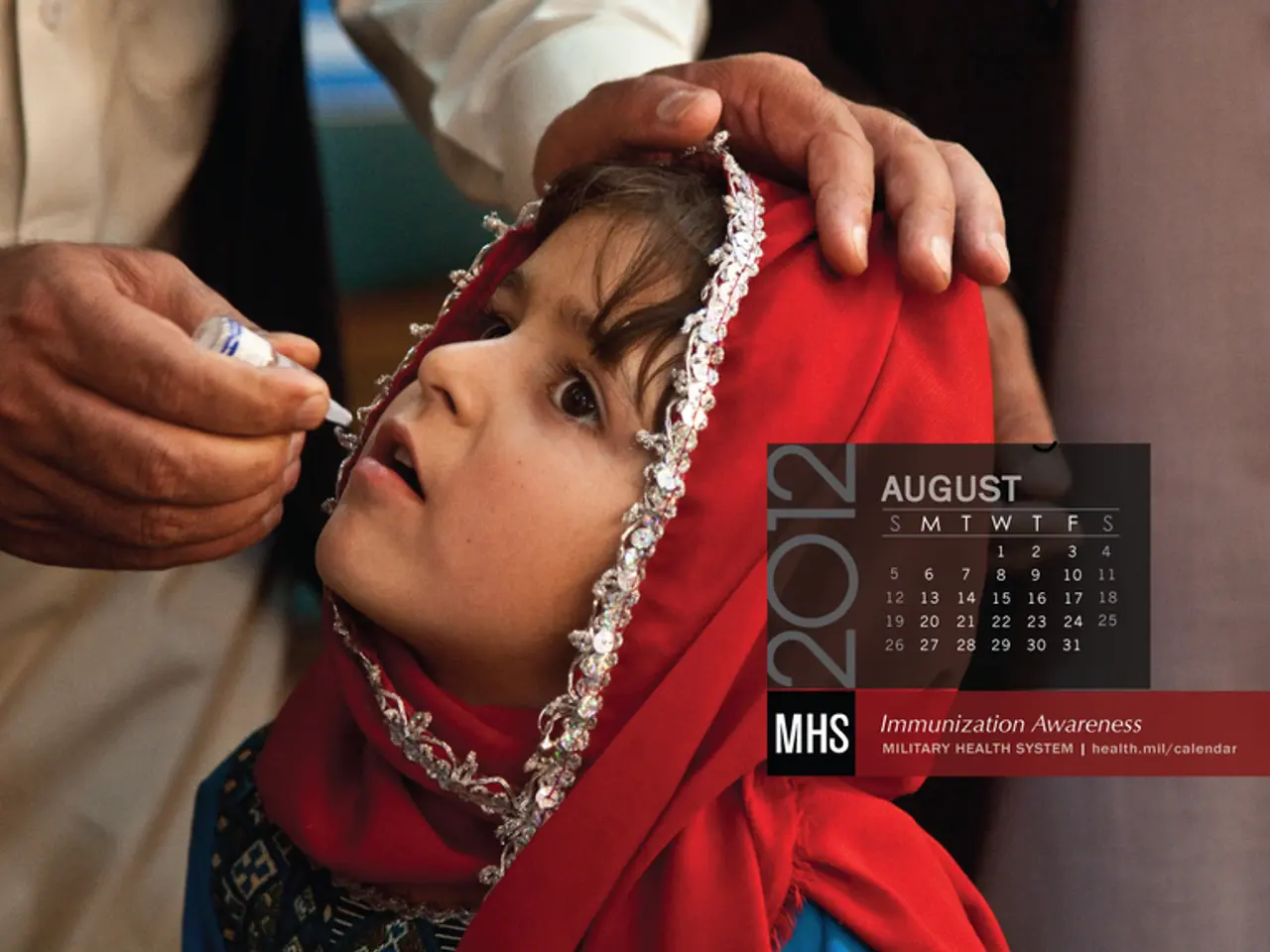
The future of the hepatitis B birth dose vaccine is under debate in the US. Anti-vaccine activists have questioned its necessity, while health experts warn that removing it could put newborns at risk. The issue has gained urgency as the Advisory Committee on Immunization Practices (ACIP) reviews long-standing vaccine policies.
The hepatitis B vaccine has been given to newborns within 24 hours of birth since 1991. Before its introduction, an estimated 18,000 infants contracted the virus each year—now, that number has dropped to around 20. The vaccine acts as a safety net, protecting babies from infected family members who may not know they carry the virus.
The ACIP has not yet altered its hepatitis B recommendations, but ongoing reviews could lead to changes. Any shift in policy may affect vaccine access and public confidence. The outcome will also depend on how the newly appointed committee members approach long-established vaccine science.