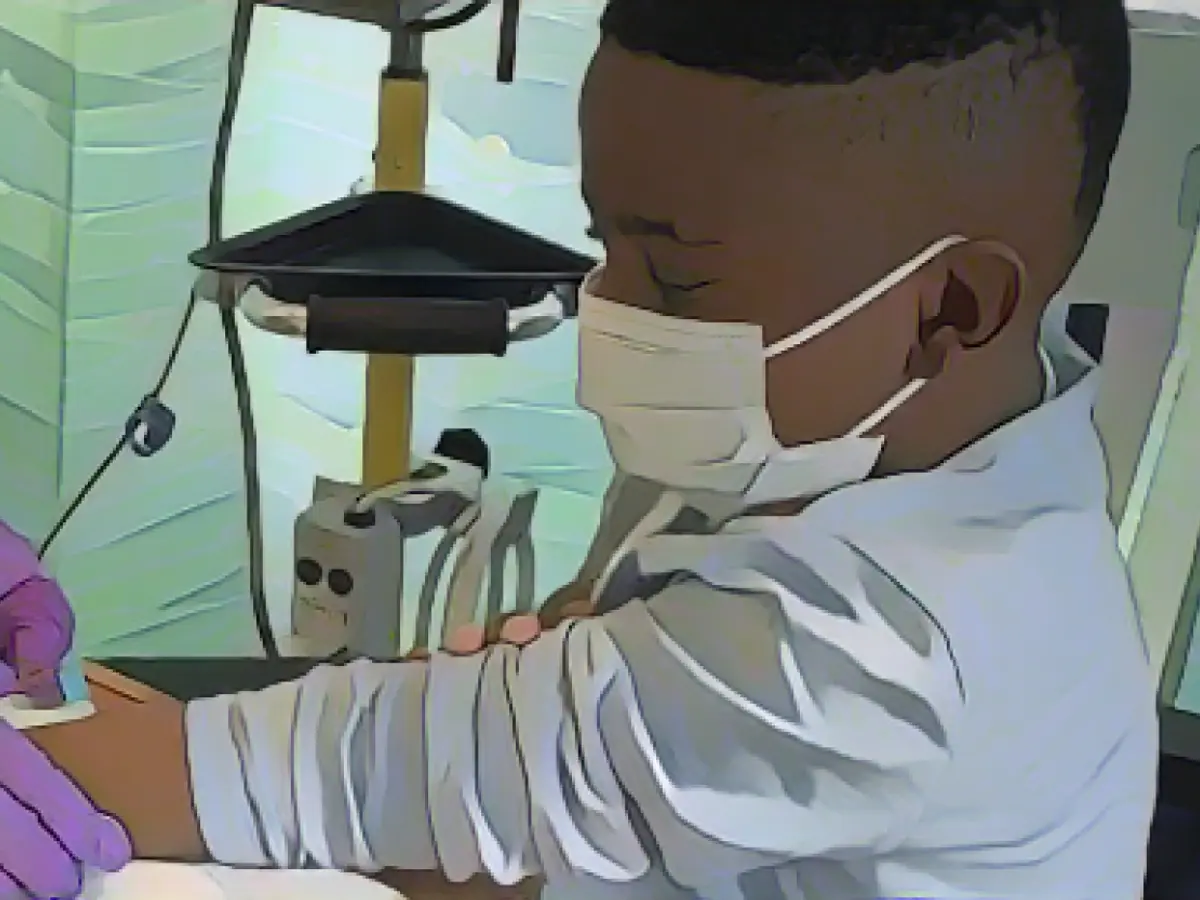
Sickle cell anemia patients in the US are eagerly awaiting the approval of a new CRISPR gene-editing therapy that could transform their lives. The treatment, known as Exa-Cel, is expected to be given the green light by the Food and Drug Administration (FDA) soon. For many in the sickle cell community, this has been a long time coming. According to the Centers for Disease Control and Prevention, roughly 1 in 365 black babies are affected by the disease, which mainly affects people of African descent. Approximately 20,000 people in the US suffer from a form of the disease severe enough to warrant this kind of treatment.
The groundwork for understanding sickle cell anemia dates back almost three-quarters of a century. The disease was first identified in 1949 by chemist Linus Pauling, who described the differences between hemoglobin in sickle cell patients and healthy individuals. This marked the beginning of molecular medicine.
"We've been waiting since DNA was first discovered," said Dr. Lewis Hsu, head of the American Sickle Cell Association and a physician treating children with the disease. "It's been a long time."
Sickle cell anemia is caused by a genetic mutation that deforms red blood cells into shapes like a crescent or sickle, such as half-moons or crescent shapes. These deformed cells can stick to blood vessels and cause damage, leading to the typical symptoms of sickle cell anemia: severe, persistent pain, known as a crisis, that can last for days.
Prior to this new treatment, the only hope for a cure for sickle cell anemia patients had been a bone marrow or stem cell transplant. However, over 80% of patients cannot find a suitable donor.
Since receiving a single infusion of his genetically-modified cells in October 2021, 15-year-old Johnny has been crisis-free.
"He was like a normal kid," said Fabienne Desir, Johnny's mother. "It changed our lives."
CRISPR is a gene-editing tool that allows scientists to precisely cut DNA. The first scientific paper on the technology was published in 2012, just eight years after it was developed by Jennifer Doudna and Emmanuelle Charpentier - who later went on to win the Nobel Prize in Chemistry.
In the case of sickle cell anemia, patient cells are removed from the body and treated with CRISPR to restore the production of fetal hemoglobin, a protein produced in the fetus that has higher oxygen-carrying capacity than adult hemoglobin. When the modified cells return to the body, the fetal hemoglobin can compensate for the mutated hemoglobin that causes sickle cell anemia.
"We know that fetal hemoglobin has a higher oxygen-carrying capacity than adult hemoglobin or sickle hemoglobin," explained Dr. Monica Bhatia, head of pediatric stem cell transplantation at Irving Medical Center of NewYork-Presbyterian/Columbia University, who was involved in the study Johnny participated in.
This new treatment could be a game-changer for sickle cell anemia patients who currently rely on transplants to manage the disease. But it comes at a price. Gentherapy treatments can cost over a million dollars and require specialized medical infrastructure.
"I don't know if it'll be covered and reimbursed," said Hsu. He added that academic medical centers that offer the treatment are usually located in large cities, making it difficult for patients in rural areas to access.
The CRISPR therapy, known as Casgevy in other countries where it's approved, is not the only new treatment option for sickle cell anemia. Another alternative, developed by Bluebird Biotech, uses a different method and is only a few weeks behind in the approval process.
"That's a huge advantage for patients," said Andrew Obenschein, CEO of Bluebird.
The effectiveness of this new treatment has the potential to be life-changing, but the cost and accessibility are major concerns.
Sources: , , , ,
Forward to a friend
Have you subscribed to CNN Health's weekly newsletter?
Subscribe now for stories from Dr. Sanjay Gupta and the CNN Health team each Tuesday.
Enrichment Data
The first scientific article on CRISPR was published in the journal Science in 2012[1][4]. Since its discovery, CRISPR has been used in a variety of applications, including gene editing, diagnostics, and therapeutics[2][5]. In sickle cell anemia therapy, CRISPR is used to edit the beta-globin gene, which produces hemoglobin[3][4]. The gene-edited cells are then infused back into the patient, allowing the production of fetal hemoglobin, which has higher oxygen-carrying capacity than adult hemoglobin[3][4].
The FDA approved Exa-Cel, or SEP-2141, for the treatment of sickle cell disease in patients aged 12 and older on December 21, 2023[1]. It is the first CAR-T cell therapy to be approved for sickle cell disease and works by modifying patients' own immune cells to fight off the disease[2]. The therapy involves withdrawing white blood cells from a patient, treating them with CRISPR-Cas9 gene editing to remove a specific gene associated with the illness, and then reinjecting them back into the patient[2].
The approval of Exa-Cel could dramatically improve the quality of life for those with sickle cell disease, as it is expected to result in significantly fewer hospitalizations and less severe disease episodes[3]. However, the cost of the therapy is expected to be high, with estimates ranging from $300,000 to $1 million per treatment course[4].